THE BATTERY SERIES
PART 2: OUR ENERGY PROBLEM: PUTTING THE BATTERY IN CONTEXT
The Battery Series is a five-part infographic series that explores what investors need to know about modern battery technology, including raw material supply, demand, and future applications.
In Part 1, we examined the evolution of battery technology. In this part, we examine what batteries can and cannot do, and the energy problem that humans hope that batteries can help solve.
Batteries enable many important aspects of modern life.
They are portable, quiet, compact, and can start-up with the flick of a switch. Importantly, batteries can also store energy from the sun and wind for future use.
However, batteries also have many limitations that prevent them from taking on an even bigger role in society. They must be recharged, and they hold a limited amount of energy. A single battery cycle is only so long, and after many of them they begin to lose potency.
Therefore, to understand the market for batteries and how it may look in the future, it is essential to understand what a battery can and cannot do.
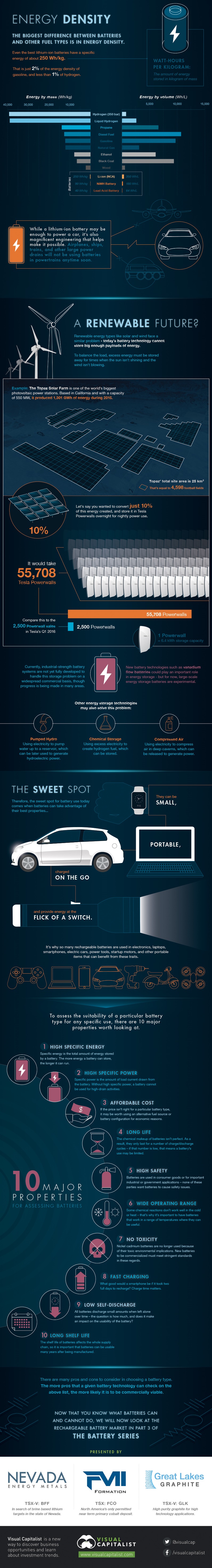
ENERGY DENSITY
The biggest difference between batteries and other fuel types is in energy density.
Even the best lithium-ion batteries have a specific energy of about 250 Wh/kg. That is just 2% of the energy density of gasoline, and less than 1% of hydrogen.
While it may be enough to power a car, it’s also magnificent engineering that helps makes this possible. Airplanes, ships, trains, and other large power drains will not be using batteries in powertrains anytime soon.
A RENEWABLE FUTURE?
Renewable energy sources like solar and wind face a similar problem – today’s battery technology cannot store big enough payloads of energy. To balance the load, excess energy must be stored somehow to be used when the sun isn’t shining and the wind isn’t blowing.
Currently, industrial-strength battery systems are not yet fully developed to handle this storage problem on a widespread commercial basis, though progress is being made in many areas. New technologies such as vanadium flow batteries could play an important role in energy storage in the future. But for now, large-scale energy storage batteries are experimental.
Other energy storage technologies may also solve this problem:
-- Chemical storage: Using excess electricity to create hydrogen fuel, which can be stored.
-- Pumped hydro: Using electricity to pump water up to a reservoir, which can be later used to generate hydroelectric power.
-- Compressed air: Using electricity to compress air in deep caverns, which can be released to generate power.
Solving this energy storage problem will pave the way for more use of renewables in the future on a grander scale.
THE SWEET SPOT
Therefore, the sweet spot for battery use today comes when batteries can take advantage of their best properties. Batteries can be small, portable, charged on the go, and provide energy at the flick of a switch.
It’s why so many rechargeable batteries are used in: electronics, laptops, smartphones, electric cars, power tools, startup motors, and other portable items that can benefit from these traits.
To assess the suitability of a particular type for any specific use, there are 10 major properties worth looking at:
-- High Specific Energy: Specific energy is the total amount of energy stored by a battery. The more energy a battery can store, the longer it can run.
-- High Specific Power: Specific power is the amount of load current drawn from the battery. Without high specific power, a battery cannot be used for the high-drain activities we need
-- Affordable Cost: If the price isn’t right for a particular battery type, it may be worth using an alternative fuel source or battery configuration for economic reasons
-- Long Life: The chemical makeup of batteries isn’t perfect. As a result, they only last for a number of charge/discharge cycles – if that number is low, that means a battery’s use may be limited.
-- High Safety: Batteries are used in consumer goods or for important industrial or government applications – none of these parties want batteries to cause safety issues.
-- Wide Operating Range: Some chemical reactions don’t work well in the cold or heat – that’s why it’s important to have batteries that work in a range of temperatures where it can be useful.
-- No Toxicity: Nickel cadmium batteries are no longer used because of their toxic environmental implications. New batteries to be commercialized must meet stringent standards in these regards.
-- Fast Charging: What good would a smartphone be if it took two full days to recharge? Charge time matters.
-- Low Self-Discharge: All batteries discharge small amounts when left alone over time – the question is how much, and does it make an impact on the usability of the battery?
-- Long Shelf Life: The shelf life of batteries affects the whole supply chain, so it is important that batteries can be usable many years after being manufactured.
There are many pros and cons to consider in choosing a battery type. The more pros that a given battery technology can check off the above list, the more likely it is to be commercially viable.
Now that you know what batteries can and cannot do, we will now look at the rechargeable battery market in Part 3 of the Battery Series.
Read more at the original source: http://www.visualcapitalist.com/our-energy-problem-battery-context/